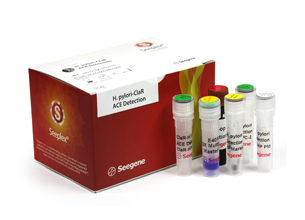
 Seeplex® H.pylori ClaR ACE Detection
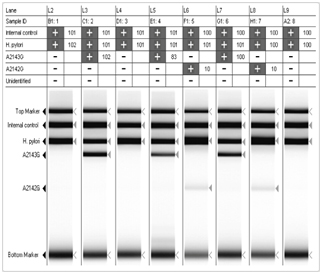
Seeplex® H.pylori ClaR ACE DetectionHelicobacter Pylori, a major cause of chronic gastritis, is strongly associated with the development of gastric and duodenal ulcers and has been linked with gastric adenocarcinoma and B-cell mucosa-associated lymphoid tissue lymphoma. Current treatment of H. pylori infection consists of a triple or quadruple regimen that includes antibiotics and a proton inhibitor, such as amoxicillin and clarithromycin. Among these, the presence of clarithromycin-resistant H. pylori has been found, which has presented a serious obstacle to the treatment of H. pylori using clarithromycin. Thus, the detection of clarithromycin-resistant H. pylori is needed to increase the efficiency of the treatment and to prescribe other antibiotics by diagnosing the presence of clarithromycin-resistant H. pylori before treatment with antibiotics. Most clarithromycin- resistant H. pylori have point mutation at the 2142 and 2143 base sequences of the 23S rRNA gene, and exist as A2142G and A2143G, respectively. Seeplex® H. pylori-ClaR ACE Detection is developed to detect these two kinds of point mutations specifically using DPO™ technology.
 Direct examination of gastric biopsy tissue without going through bacterial culture
Direct examination of gastric biopsy tissue without going through bacterial culture
 Simultaneous identification of H. pylori and two point mutations (A2143G & A2142G), which cause clarithromycin
Simultaneous identification of H. pylori and two point mutations (A2143G & A2142G), which cause clarithromycin
resistance without culture
 Applicable for Auto-capillary Electrophoresis instruments
Applicable for Auto-capillary Electrophoresis instruments
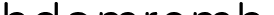 A single round PCR process reduces the carryover contamination risk, and 8-MOP, which is included in product,
A single round PCR process reduces the carryover contamination risk, and 8-MOP, which is included in product,
eliminates the contamination risk from PCR products
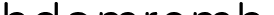 Accurate system for result interpretation included Internal Control and Positive Control
Accurate system for result interpretation included Internal Control and Positive Control
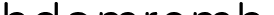 H.pylori
H.pylori
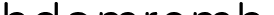 A2142G
A2142G
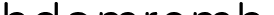 A2143G
A2143G
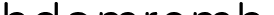 Gastric biopsy
Gastric biopsy